The Clinical Cannabis Initiative
The Clinical Cannabis Initiative
We are surrounded by information about cannabis and cannabinoid products from anecdotes and studies without nearly enough time to sort through it all. Thankfully, The Board of Medicine is here to help! The Board of Medicine is a group of expert physician specialists, scientists, and subject matter experts who have evaluated all of the available peer-reviewed data to date on cannabinoids (such as CBD and THC) for medical purposes. We have compiled this knowledge into one of the first comprehensive evidence-based peer-reviewed clinical guidelines for the safe use of medical cannabis and cannabinoid products. The first of this series of manuscripts was published in 2023 describing the evidence-based treatment guidelines for minimizing harm when managing opioid dependence and chronic pain using cannabinoids entitled: An answered call for aid? Cannabinoid clinical framework for the opioid epidemic in Harm Reduction Journal.
Our Clinical Cannabis Initiative also provides peer-reviewed evidence-based guidelines, trainings, and certifications for the safest and highest quality cannabis products, cultivators, processors, dispensaries, and care providers.
These updated standardized guidelines are essential in public health. Not only has the importance of the endocannabinoid system been well recognized by the scientific community, but it may help to explain a number of chronic illnesses, such as chronic intractable pain, that are notoriously difficult to treat. In a 2018 JAMA article reviewed by Dr. Peter Grinspoon MD for Harvard Medical School, a longitudinal analysis of the number of opioid prescriptions filled under Medicare Part D, showed that when medical marijuana laws went into effect in a given state, opioid prescriptions fell by 2.21 million daily doses filled per year from an average of over 23 million daily doses per year. When medical marijuana dispensaries opened, prescriptions for opioids fell by 3.74 million daily doses per year. The reductions in opioid prescribing were particularly notable for hydrocodone (Vicodin) and morphine prescriptions, but these reductions also extended to benzodiazepines, stimulants, and many mental health medications that are known to be over-prescribed.
All of this being said, the use of cannabis and cannabinoid products is not without risk. Interestingly, most of the side effects from using cannabinoid products appear to arise from contaminated products, mislabeled products, and people not knowing what products are appropriate for their needs, or how to dose them correctly. With a focus on harm-reduction, The Board of Medicine has made it a priority to provide evidence-based guidelines, trainings, and certifications for the production and use of cannabinoids and other safe plant medicine products to minimize potential risks and maximize potential benefits.
The Board of Medicine also provides expert research consultation to improve study quality and clinical care. We train and certify physicians and care providers.
Trainings and certifications are based on evidence collected from a comprehensive review of the peer-reviewed literature on cannabis and cannabinoids combined with evidence from thousands of anecdotal reports from patients and providers. These guidelines focus on harm-reduction and education about how to use cannabinoids safely for improving treatment outcomes and how to avoid unwanted effects.
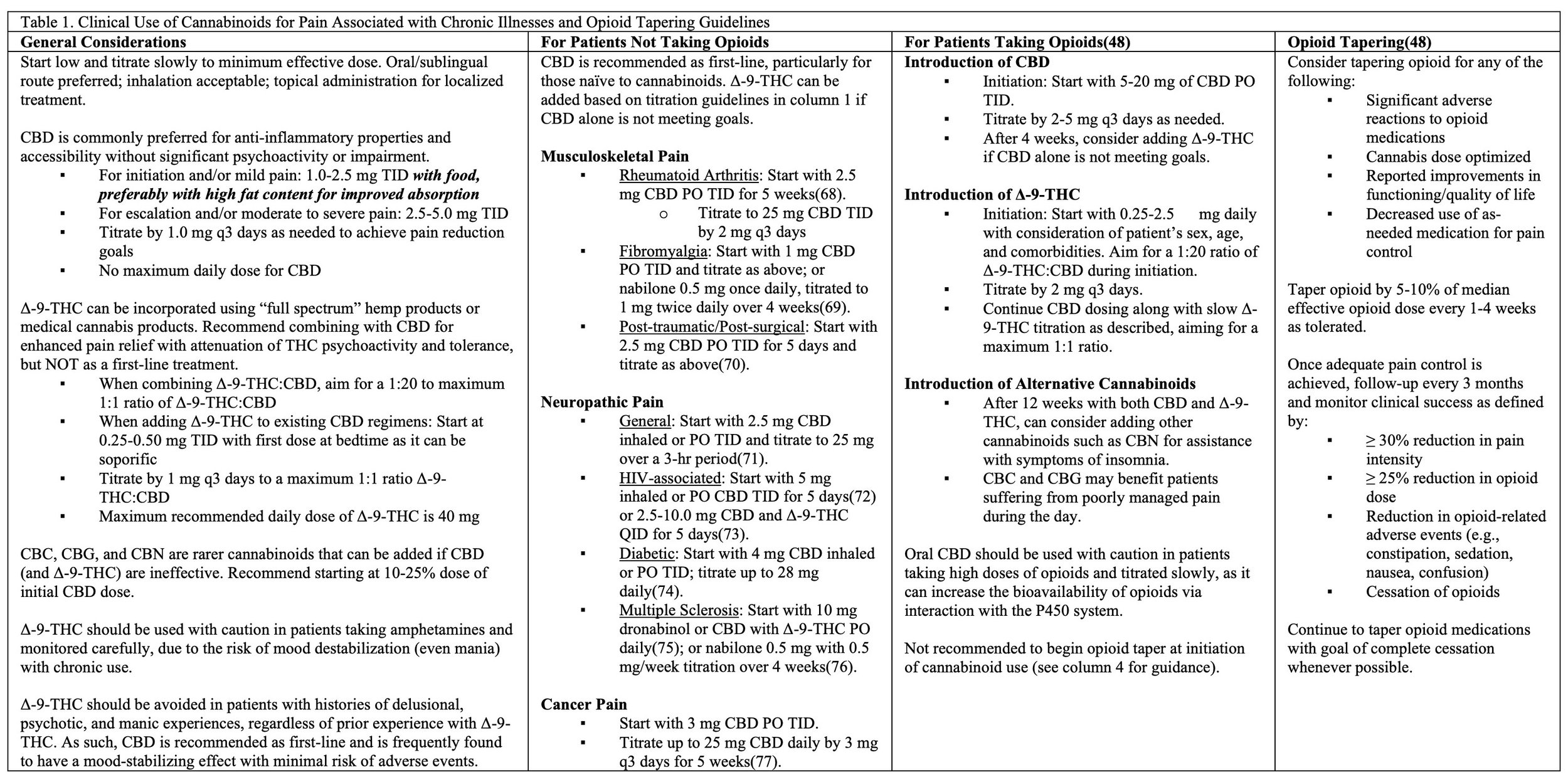
In 2023, the Board of Medicine team published some of the world’s first comprehensive clinical guidelines recommending evidence-based treatment programs for improving harm-reduction when managing opioid dependence and chronic pain using cannabinoids entitled: An answered call for aid? Cannabinoid clinical framework for the opioid epidemic
In collaboration with best-in-class product manufacturers, The Board has independently verified and certified some of the first physician-approved cannabinoid products to guide clinicians and patients toward the safest available products.
Product review for certification: $1500.00 USD
Please contact us at using the form at the bottom of this page to inquire about product certification.
-

Blue Certification (Tier 3)
Third party validation stating:
No medicine should contain contaminants to include (at acceptable levels):
- Pesticides
- Herbicides
- Fungicides
- Heavy Metals
- Solvents - harmful solvents
- Fillers known to be toxic
All medicine should be what it says it is (ie. on the label) - within 10% variance
Cultivation is in compliance with state
Organic farming practices
Full panel testing -

Silver Certification (Tier 2)
Blue criteria plus:
Broad Spectrum or Full Spectrum (No isolate)
Products sourced from USA
Full panel testing - including terpene profile
SOPs for farming and production for reproducible processes -

Gold Certification (Tier 1)
Silver criteria plus:
Known source of seed & stable genetics
Organic, Regenerative farming practices
Flower used in extraction
Full spectrum
Subcannabinoids and wide spectrum of terpenes and flavonoids present
Subcritical CO2 or ethanol extraction
Verified educational content
The Board of Medicine Certified Cannabinoid Products
-

Artis Botanticals
Certified Gold
2020-2023
-

Onda Wellness
Certified Silver
2022-2023
-

Earth to Mind
Certified Silver
2021-2023
-

CV Sciences
Certified Blue
2022-2023
-

Ojai Energetics
Certified Blue
2022-2023
Contact us.
Info@theboardofmedicine.org


